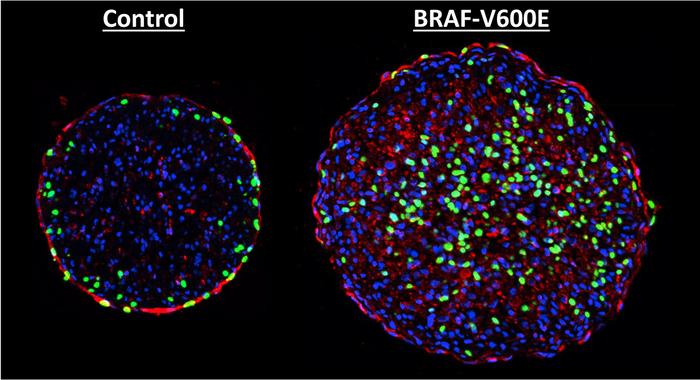
BRAF mutations linked to aggressive melanomas have been shown to induce rat heart tissue growth in vitro, suggesting a potential therapeutic approach to rebuilding human heart muscle after a heart attack.
Duke University researchers studied neonatal rat heart cells grown within a 3D hydrogel environment. The specially developed hydrogel provides cues to grow and mature cells into adult-like heart muscle tissues, when cell division naturally stops. They then infected the tissue with a virus loaded with a mutated BRAF gene. As expected, the virus inserted the mutated gene into the cells and became part of the cells’ DNA. When researchers introduced a drug that caused the mutated BRAF genes to activate, heart muscle cells entered DNA synthesis— the first step in cell division and growth.
While promising, the results of the study published in Science Advances also indicate cause for concern. Once cells began multiplying, the tissue as a whole lost 70% of its contractile strength, researchers said.
Before progressing to human studies, researchers will need to precisely control the dosage and duration of gene activation. This will include identifying a new gene delivery system. Methods such as lipid nanoparticles and short-living viruses are being developed, but neither is ready for human application. Researchers also seek to understand how they may instigate heart tissue regeneration without causing the tissue to lose strength.
Studies in live animals are being planned with a goal toward better understanding what other genes and processes are activated by the mutated BRAF gene, and whether proliferation could be independently activated without functional decline in order to efficiently promote healing.